This page was generated automatically; to view the article in its initial location, please visit the link below:
https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-024-20922-x
and if you wish to have this article removed from our site kindly reach out to us
Our protocol complies with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines, with the finalized SPIRIT checklist provided in Supplementary file S1 [31]. The trial is officially recorded with the Clinical Trial Registry of India [(CTRI/2024/10/074559) (https://www.ctri.nic.in/Clinicaltrials/login.php)].
Study design
We will implement a two-arm, parallel-group, community-based, multi-centre, cluster randomized controlled trial featuring a 1:1 allocation ratio during the study period of 2025-26. This investigation encompasses an intervention group receiving the 6-month MultiLife mHealth program alongside a control group that receives standard care.
The trial employs a Type 1 hybrid effectiveness-implementation design, which evaluates the impact of the MultiLife intervention while concurrently collecting data on its implementation process [32, 33]. This hybrid methodology is particularly advantageous for examining clinical outcomes in conjunction with vital implementation factors such as acceptability, adoption, fidelity, and maintenance within actual primary healthcare environments. We will utilize a mixed-method strategy. Quantitative techniques will evaluate clinical outcomes, including HbA1c, blood pressure (BP), and BMI. Qualitative techniques will gather insights from patients and healthcare personnel regarding the intervention’s effect and feasibility of implementation.
Theoretical framework
Behavioral change theories are crucial for creating interventions that encourage enduring health behavior adjustments. The Health Belief Model (HBM) acts as the theoretical framework for our study [34]. HBM offers a systematic insight into how patient beliefs and perceptions affect their health behaviors and how the intervention can reshape these beliefs to foster improved health outcomes [35]. The MultiLife intervention is anchored in six fundamental HBM constructs:
-
1.
Perceived Susceptibility: Patients need to recognize that they are quite vulnerable to complications like Acute Myocardial Infarction or stroke due to the coexistence of numerous cardiometabolic conditions. The MultiLife app will provide educational content highlighting the risks connected with unmanaged CMM, instilling a sense of urgency for lifestyle adjustments and adherence to prescribed medications.
-
2.
Perceived Severity: If patients are convinced that the repercussions of uncontrolled diabetes or hypertension are serious, they may be more inclined to participate in preventive actions. The MultiLife app will feature videos demonstrating the potential outcomes of unmanaged blood sugar and BP, such as kidney failure, vision impairment, or cardiovascular incidents, making the dangers feel more immediate and relatable.
-
3.
Perceived Benefits: Success narratives and actual instances of reduced HbA1c levels, BP, and BMI will be shared with participants to emphasize the practical advantages of their efforts. Participants will have the opportunity to monitor enhancements in their health metrics (e.g., HbA1c levels, BP, BMI), solidifying the perceived benefits of the intervention.
-
4.
Perceived Barriers: Barriers may encompass a lack of time, challenges in accessing nutritious foods, or apprehension regarding side effects from medications. The app will offer practical, culturally tailored solutions such as meal plans and straightforward physical activity recommendations that can be performed at home. ASHA workers will provide assistance during regular home visits to address any questions.
-
5.
Cues to Action: Daily prompts and alarms will function as cues to action, encouraging participants to take their medications, engage in physical activities, maintain a nutritious diet, and refrain from tobacco and alcohol consumption.
-
6.
Self-Efficacy: The app will feature functionalities that enable patients to establish realistic short-term goals (e.g., completing a 10-minute walk daily or eliminating sugar from one meal), track their progress, and earn rewards or recognition for their achievements.
Study location
The study will take place in Odisha and Jharkhand, two eastern states of India recognized for their transition from communicable to non-communicable diseases (NCDs) [3, 36]. These states have significant tribal populations and are categorized as “high focus” states by the National Health Mission due to their inadequate public health indicators and/or infrastructure [37,38,39]. To enhance generalizability, we selected the three most densely populated districts—two in Odisha (Cuttack and Ganjam) and one in Jharkhand (Ranchi)—which represent diverse cultural and socioeconomic contexts yet face comparable health challenges. The study will concentrate on three blocks within these districts: Sheragada in Ganjam (Odisha), Tigiria in Cuttack (Odisha), and Namkum in Ranchi (Jharkhand) (Fig. 1). These areas report a higher incidence of CVDs, diabetes, and other chronic illnesses relative to the national average, rendering them appropriate for investigating CMM management [40,41,42].
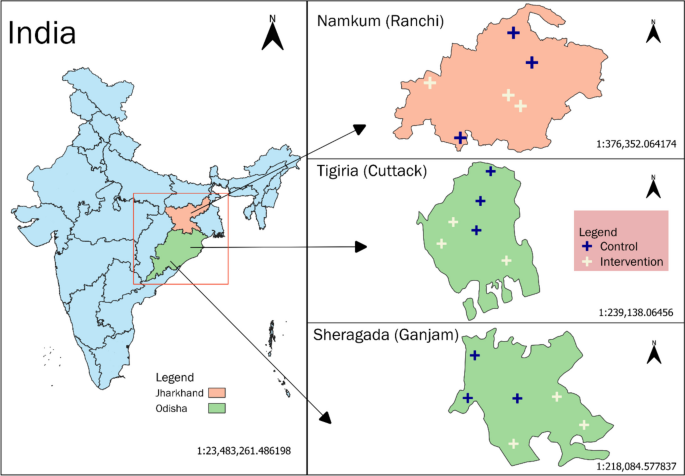
The various research locations for assessing the MultiLife initiative in Eastern India.
In these areas, primary health facilities functioning under the Ayushman Bharat initiative are known as Ayushman Arogya Mandir – Health and Wellness Centres (AAM-HWCs) [43]. Their function in managing NCDs encompasses screening, counselling, basic treatment, and follow-up care for ailments like hypertension and diabetes, in addition to offering essential diagnostics and medications [44]. In every AAM-HWC, Healthcare Workers (HCWs) are pivotal in NCD management. ASHAs, referred to as Sahiyas in Jharkhand, execute community outreach, assess risk factors, and direct individuals aged 18 + to AAM-HWCs for screenings [45, 46]. Auxiliary Nurse Midwives (ANMs) conduct screenings for NCDs, facilitate medication adherence, and provide referral assistance. Community Health Officers (CHOs) stationed at AAM-HWCs deliver primary care, oversee screenings, diagnose uncomplicated conditions, initiate treatment, and coordinate referrals to higher-tier facilities [47, 48]. This collaborative healthcare framework promotes early detection, prompt referrals, and management of NCDs, serving as the foundation for integrated CMM care.
In our research, each location consists of AAM-HWCs as detailed below:
-
Sheragada Block (Ganjam): Eight AAM-HWCs, catering to 8,844 households across 32 villages, with 2,433 individuals recorded with CVD or diabetes.
-
Tigiria Block (Cuttack): Twelve AAM-HWCs, serving 14,844 households across 49 villages, with 3,203 individuals documented with CVD or diabetes.
-
Namkum Block (Ranchi): Twenty-five AAM-HWCs, serving 21,166 households across 93 villages, with 6,064 individuals noted with CVD or diabetes.
Among the 45 AAM-HWCs, 18 will be randomly assigned as clusters into either the intervention or control group.
Eligibility criteria
Cluster eligibility
AAM-HWCs are designated as the clusters for this research. Only those AAM-HWCs that have employed ASHAs and ANMs for a minimum of three months will qualify to participate.
Patient eligibility
Inclusion Criteria
-
Age: Individuals who are 18 years and older.
-
Diagnosis: Individuals with a confirmed diagnosis of CMM, defined as diabetes mellitus accompanied by either hypertension, coronary heart disease, or a history of stroke.
-
Access to a smartphone for installing and using the app.
The diagnosis of diabetes will rely on previous clinical diagnoses or current use of anti-diabetic medications [49]. Hypertension will be confirmed similarly, established through past clinical diagnosis or ongoing use of antihypertensive drugs [50]. Heart disease encompasses conditions such as coronary artery disease, heart failure, and arrhythmias, verified through self-reporting and prescription documentation. Stroke will be identified through self-reported diagnoses, supplemented by medical records or prescriptions indicating neurological impairments.
Exclusion Criteria
Pregnant women, patients with cognitive disabilities impairing the ability to follow instructions, and individuals who do not plan to stay in the study area during the intervention period.
Participant withdrawal
We expect a minimal withdrawal rate, as participants are regular patients frequently visiting the centres for medications and routine check-ups.
Intervention
We will design the MultiLife mobile application to aid patients with CMM in adopting healthier habits and managing diabetes and hypertension via structured daily reminders and health education (HE) broadcasts. Through consultations among specialists in NCDs, digital health experts, IT professionals, and healthcare stakeholders, we identified and crafted core elements aimed at increasing participant engagement in behavioral modification and medication adherence. Features of the MultiLife App:
-
Reminder System: Participants will receive daily prompts and alarms each morning from the application to facilitate behavior change. These reminders will include notifications to take prescribed medications, practice yoga daily, fulfill daily challenges (such as walking 8,000 steps and specific exercise routines), maintain a proper diet, and avoid tobacco and alcohol consumption. Alerts are accompanied by sound signals and can be customized based on user preferences.
-
HE Broadcast: Short educational videos, grounded in the HBM and WHO-HEARTS framework, will be provided through the app. Each week, on a chosen day decided by the participants (e.g., every Tuesday or Wednesday), a 1–2-minute video featuring audio and subtitles in the local language will be released. To access the subsequent video, participants must complete each weekly session within the designated timeframe. If a session remains unfinished, the app will retain the current video until the participant views it, ensuring each educational step is accomplished sequentially.
-
Video Timeline and Structure: The intervention is designed around a 6-week cycle of HE sessions, each concentrating on one of the six HBM components: perceived susceptibility, perceived severity, perceived benefits, perceived barriers, cues to action, and self-efficacy. Following this initial 6-week series, videos will recur weekly for the remaining 6-month intervention period, reinforcing essential behaviors.
This iterative methodology guarantees that every participant will experience at least one comprehensive series of videos by the conclusion of the study (T6).
-
Curated Material: Instead of generating original material, we will meticulously gather publicly accessible HE videos, chosen through formative research to ensure their cultural and contextual relevance to the target demographic [51]. The curated material will concentrate on lifestyle practices essential for managing CMM, with detailed information provided in Supplementary file S2.
-
-
Engagement Monitoring: The application will monitor participants’ involvement, such as viewing reminders and engaging with HE material. Activity records will be compiled to produce participation summaries for each individual, allowing project staff to oversee their engagement.
-
Online Dashboard: A singular platform for aggregating data from the application to enable real-time observation and evaluation by project personnel, offering insights into the level of adherence of participants to the intervention.
-
User Orientation: A 30-minute orientation will be given to all users, including patients, HCWs, and other healthcare personnel, to facilitate smooth integration and proficient utilization of the application by the project team.
Pilot evaluation and validation
Prior to large-scale rollout, we will conduct a pilot evaluation of the MultiLife application with 10–20 CMM patients and 3–4 HCWs to gauge its usability, relevance of content, and operational capabilities. Insights derived from this assessment will direct final refinements to the app’s design and material. Initially, we will instruct ASHAs and ANMs to aid in app utilization. Subsequently, they will register eligible participants and assist them in installing and navigating the application’s features. Once enrolled, the app will send daily reminders and HE broadcasts to the participants. We will host one focus group discussion (FGD) and 2–3 in-depth interviews (IDIs) with the pilot participants and HCWs to collect input regarding app usability, content significance, and possible enhancements. Modifications will be made to the app’s user interface, reminders, and.
HE material will be based on this feedback. Participants in the pilot will not engage in the trial to eliminate bias.
Intervention and control groups
Intervention Group
Those in the intervention section will receive the MultiLife intervention over a six-month period. To assure the efficiency and sustainability of the intervention, it will be facilitated by ASHAs and ANMs. They will undergo training to execute the intervention, grasping both its theoretical framework and practical procedures. Training sessions will encompass intervention aims and protocol specifics. Project personnel will evaluate the preparedness of the ASHAs and ANMs, who will partake in supervised practice sessions to tackle potential obstacles. Within the intervention group, participants will receive:
-
Daily reminders for medications, physical exercises, dietary habits, and HE conveyed through the MultiLife app.
-
Ongoing access to routine healthcare provided by CHOs at AAM-HWCs. Standard care includes regular health assessments, lifestyle recommendations, and necessary prescriptions in accordance with the National Programme for Prevention and Control of NCD (NP-NCD) guidelines [52,53,54]. Patients typically receive a one-month supply of medication, while stable patients may qualify for a three-month supply [54]. CHOs also offer health education on lifestyle adjustments to reinforce the significance of adhering to medications and effecting behavioural changes.
Control group
Outcomes
Primary outcome measure
The primary outcome aims to evaluate the clinical efficacy of the MultiLife intervention by observing changes in HbA1c levels from baseline (T0) to six months (T6). A reduction of at least 0.5% points in HbA1c will be deemed clinically significant. HbA1c will be assessed at three time points: T0, T3, and T6 by accredited clinical research personnel (e.g., phlebotomists or medical assistants). Blood samples will be taken from both intervention and control participants and centrifuged within one hour at the collection site. The serum will be transferred to labeled vials and preserved at −20 °C prior to being sent to the National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited labs at each study location [55]. Biochemical assays will be conducted using the same liquid chromatography technique across various sites during the study period.
Secondary outcome measures
We will assess the following clinical, behavioural, and quality-of-life metrics in both intervention and control groups.
-
a)
Blood Pressure: BP will be measured while seated using the Omron HEM-7124 device, which will have undergone prior validation on 20 subjects. BP will be checked before blood sampling to alleviate anxiety and will adhere to the WHO STEPS protocol [56]. Participants will be instructed to refrain from exercise, food, alcohol, and smoking for 30 minutes prior to the reading.
-
b)
Anthropometrics: Height, weight, and waist circumference will be recorded using standardized methods [57]. BMI will be calculated as weight (in kilograms) divided by the square of height (in meters).
-
c)
Physical Activity: Sedentary behaviours and levels of physical activity will be evaluated using the Madras Diabetes Research …Foundation-Physical Activity Questionnaire (MPAQ), which encompasses physical activity within occupational, recreational, and transportation areas. This instrument is confirmed for utilization in both rural and urban environments in India [58].
-
d)
Dietary Trends: Modifications in dietary behaviors will be evaluated utilizing the Mini-Eating Assessment Tool (Mini-EAT), a 9-item screening tool that gauges the consumption of fruits, vegetables, grains, dairy, fish, legumes, and desserts [59]. It offers a rapid evaluation of dietary quality and shifts in the Healthy Eating Index (HEI).
-
e)
Life Quality (QoL): QoL will be assessed through the EuroQol Quality of Life India Questionnaire (EQ-5D). This instrument examines dimensions such as mobility, self-care, typical activities, pain/discomfort, and anxiety/depression. It features a Visual Analogue Scale (EQ-VAS), allowing participants to rate their overall health from 0 to 100 [60].
-
f)
Tobacco and Alcohol Consumption: The WHO Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) will evaluate tobacco and alcohol usage. This validated instrument classifies participants based on their risk levels concerning substance-related health issues [61].
-
g)
Medication Compliance: We will evaluate medication compliance using the 4-item Morisky Medication Adherence Scale (MMAS-4). Participants will answer four yes/no questions, producing scores that range from 0 (high compliance) to 4 (low compliance). This instrument is effective in monitoring adherence to prescribed medications over the long term [62].
Implementation outcome assessment
Implementation outcomes will be examined utilizing the RE-AIM Qualitative Evaluation for Systematic Translation (RE-AIM QuEST), a mixed methods methodology [63]. The evaluation strategy incorporates both quantitative and qualitative assessments, as detailed in Table 1.
Participant timeline
The MultiLife intervention will extend over six months, with ASHAs and ANMs providing continuous support. Participants will be assessed at three intervals: T0, T3, and T6. Implementation outcomes such as feasibility and fidelity will be monitored consistently. Upon completing the intervention, all participants and healthcare professionals will fill out an exit questionnaire, with some engaging in IDIs and FGDs to evaluate the intervention’s acceptability and adoption. A follow-up survey three months after the intervention will assess the sustainability of the outcomes. The project timeline and SPIRIT flowchart are illustrated in Fig. 2.
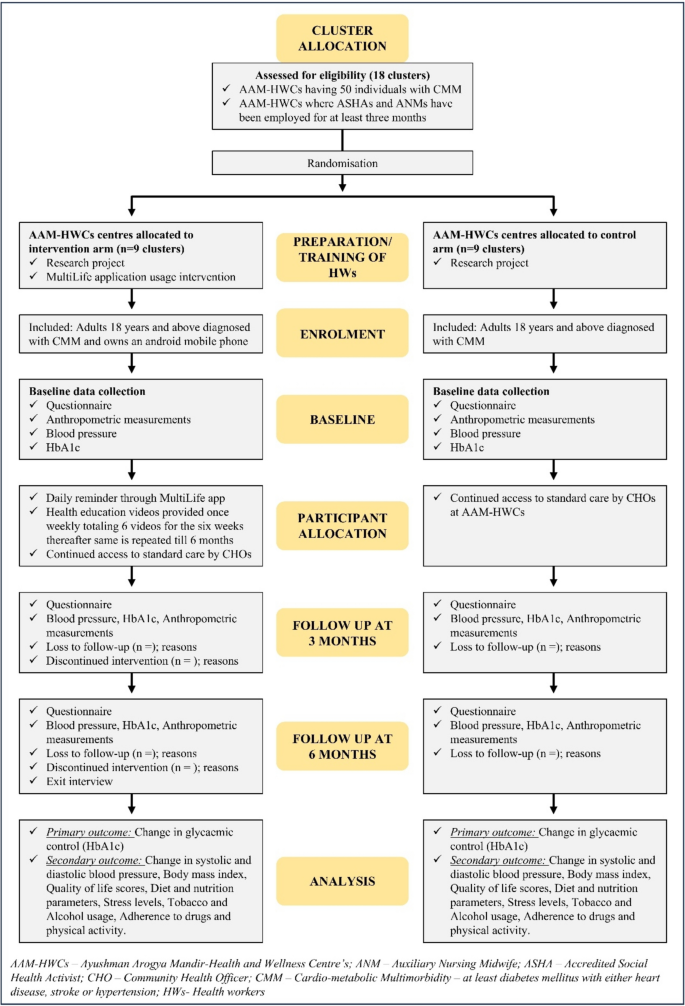
Flowchart for MultiLife intervention versus standard care services in diabetes and hypertension management among individuals with cardiometabolic multimorbidity
Sample size
The sample size determination is founded on a two-group cluster design comparative analysis, with the following parameters: anticipated decrease of 0.5 units in HbA1c across both arms [64], a standard deviation of 1, cluster size of 50, design effect of 2, power of 80%, alpha error of 5% (two-sided), and a 10% drop-out rate. Each segment of the study will require 140 participants per site (totaling 280 per site), amounting to 840 participants across three sites (3 sites x 140 participants per arm x 2 arms) [65]. The trial will be undertaken in 18 clusters, with six clusters allocated for each site.
Intervention assignment
To prevent contamination and ensure representativeness, six geographically distinct centers will be chosen for each site. Block randomization will occur at the cluster level, employing random sequences generated via Excel. Each of the 18 centers will be designated for either the intervention or the control group (three AAMs assigned to each group per site). Allocation will be centrally managed and maintained until the randomization process is finalized. Following the randomization of clusters, the HCWs of the AAMs will be notified of their respective assignments. Given the nature of the intervention, blinding of HCWs and participants is not feasible; however, an independent statistician will supervise the randomization process and verify analyses to avert bias.
Recruitment
Each AAM-HWC will operate as a cluster for recruitment purposes. ASHAs and ANMs will undergo training on the MultiLife study, preparing them to identify and recruit suitable participants during routine care visits, NCD clinic attendance, and Population-Based Screening initiatives. ASHAs and ANMs will help participants to download and install the MultiLife mobile application during clinic visits. If patients are unable to finalize installation during their visit, ASHAs will follow up with home visits to ensure app installation and provide assistance on its usage.
Training will also be extended to participants on utilizing the app independently. Recruitment and app installation processes will be thoroughly documented to keep a detailed record of participant engagement. Upon enrollment, participants will adhere to a self-monitoring model using the MultiLife app autonomously. Direct messaging and social support networks will not be featured within the app; however, participants may reach out to the study team via the “Messages from the Researchers” function for study-related inquiries or technical assistance. Routine safety monitoring will be conducted by analyzing app usage data, capturing participant interactions and any technical difficulties encountered.
Data gathering
Staff questionnaire
Upon acquiring informed consent, ASHAs and ANMs at each research site will complete a self-administered survey to gather demographic and professional information, including age, gender, educational qualifications, and relevant work experience. This data will assist in evaluating staff characteristics and their readiness for implementing the intervention.
Participant questionnaire
Post-informed consent acquisition, we will deliver a structured questionnaire to each participant to collect sociodemographic information (e.g., age, gender, educational attainment, monthly household income, marital status, and employment status) along with behavioral data. Chronic conditions will be evaluated using the Multimorbidity Assessment Questionnaire.for Primary Care (MAQ-PC) [66]. The survey will be translated into Hindi and Oriya and subsequently back-translated to English to guarantee linguistic and cultural precision. Table 2 presents a summary of variables, instruments, and collection time intervals. Participants who opt-out will be requested to fill out a brief refusal questionnaire that gathers essential information, including age, gender, recent blood testing and BP status, current medications, and reasons for non-participation. This information will enable us to evaluate the representativeness of our recruited sample by juxtaposing participant and non-participant profiles.
Data management
Considering the intricate nature of data collection across various locations and time points, we will establish a Database Management System for this research. This system will incorporate an electronic data collection tool (an Android application), a web-based portal for data oversight, a module for integration with the MultiLife intervention app, and a real-time dashboard for activity monitoring in the field. The backend of this framework will be driven by SQL (Structured Query Language), facilitating effective data management and querying. The dashboard will monitor daily data collection, participant involvement, and app utilization, providing real-time updates for field activities based on specific metrics. Access to this password-protected dashboard will be limited to lead researchers, the project manager, and the statistician, ensuring secure data management. All data gathered by the project staff will be uploaded via survey software on password-protected tablets equipped with antivirus protection. Data will comply with the ICMR National Guidelines for Data Quality in Surveys with regard to data transfer, storage, and analysis.
Statistical analysis
Continuous variables will be presented as means ± SD for data that follows a normal distribution and as medians and interquartile ranges for non-normally distributed data. Categorical variables will be described using percentages and frequencies. To evaluate baseline discrepancies between intervention and control groups, we will employ independent samples t-tests (for normally distributed data) or Mann-Whitney U tests (for non-normally distributed data) for continuous variables, alongside chi-squared or Fisher’s exact tests for categorical variables.
The principal analysis will utilize an intention-to-treat approach, analyzing participants based on their initially assigned intervention group, irrespective of adherence to the intervention [67]. Individual-level data from 18 AAMs will be analyzed, considering the clustering effect. Summaries at the cluster level will be generated for key outcomes, like the mean (SD) change in HbA1c within each AAM, and compared across intervention and control groups. Any baseline covariates significantly linked with outcomes (p < 0.05) will be included in models for primary and secondary outcomes to enhance precision and mitigate bias. We will leverage random-effects regression to conduct a difference-in-difference analysis, adjusting for covariates and accounting for within-cluster correlation at the AAM level. A repeated measures mixed-effects model will be used for both primary and secondary outcomes to monitor changes over time (T0, T3, T6). This model will encompass interaction terms between the intervention group and time to evaluate differences in trends across groups. Multiple imputation methods (MICE) will be applied to handle missing data, ensuring robust and valid conclusions [68]. All analyses will be carried out using STATA 16 (StataCorp LP, College Station, Texas, USA) and R version 3.4.4 (https://www.r-project.org/) [69, 70].
Qualitative data from FGDs and IDIs will be assessed thematically using NVivo software [71]. We will formulate a structured coding framework for the organization and classification of data. All interview recordings will be accurately transcribed, and a second researcher will independently code a portion of transcripts to verify coding consistency and reliability. Triangulation between participant and HCW responses will fortify the validity of the findings. Thematic analysis will unveil insights into intervention implementation, pinpointing barriers, facilitators, and areas for enhancement. Reporting will adhere to the Consolidated Criteria for Reporting Qualitative Studies (COREQ) guidelines [72].
Ethics and dissemination
Ethical approvals were secured from institutional ethics committees at the three study locations and the state ethics committees of Jharkhand and Odisha. All participants will be required to provide written informed consent before engaging in the study. Prior to consent, participants will be fully briefed on the study’s objectives, procedures, and potential risks. They will retain the right to withdraw from the study at any moment without adverse consequences. Consent will encompass agreement to three face-to-face interviews, blood sample collection, BP and anthropometric measurements, and optional involvement in FGDs or IDIs if necessary. The study will adhere to the ethical guidelines delineated in the ICMR National Ethical Guidelines for Biomedical and Health Research Involving Human Participants. Steps will be taken to ensure participant confidentiality throughout recruitment, data collection, transcription, and analysis. Each participant will be assigned a unique identification number, and all data will be anonymized prior to statistical evaluation. Any physical documents containing personal details will be securely stored in locked cabinets. Data will be safely stored on encrypted servers, with regular backups performed to avert data loss. Access will be limited to authorized personnel only, ensuring confidentiality throughout the study. All data will be securely stored for five years. Study findings will be disseminated through peer-reviewed publications in pertinent scientific journals, presentations at conferences, and policy briefs. The results will also be communicated to the study participants, relevant stakeholders, and state health departments to ensure practical application and policy ramifications, thereby contributing to improved CMM management in low-resource environments.
This page was generated programmatically; to read the article in its original location, you can visit the link below:
https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-024-20922-x
and if you wish to remove this article from our site, please contact us.



