This web page was created programmatically, to learn the article in its unique location you possibly can go to the hyperlink bellow:
https://www.sciencealert.com/experiment-recreates-the-universes-very-first-chemical-reactions
and if you wish to take away this text from our web site please contact us
The first chemical reactions within the wake of the Big Bang have been recreated for the primary time in situations much like these within the child Universe.
A group of physicists Led by Florian Grussie of the Max Planck Institute for Nuclear Physics (MPIK) in Germany has reproduced the reactions of helium hydride ion (HeH+), a molecule comprised of a impartial helium atom fusing with an ionized atom of hydrogen.
These are the primary steps that result in the formation of molecular hydrogen (H2); probably the most plentiful molecule within the Universe and the stuff from which stars are born. The new work, due to this fact, elucidates a few of the earliest processes that gave rise to the Universe as we all know it in the present day.
Related: The First Molecular Bond in The Universe Has Finally Been Detected in Space
 frameborder=”0″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
frameborder=”0″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>The birth throes of the Universe some 13.8 billion years ago produced a hot, dense soup of fundamental particles simmering at temperatures too high for atoms to form.
It took about 380,000 years for nuclei and electrons to lose enough energy to congeal into the very first elements. Those elements were the lightest the periodic table has to offer; about 75 percent hydrogen, 25 percent helium, and trace amounts of lithium.
Hydrogen continues to dominate the Universe’s ingredient list today, as clouds of molecular gas that give birth to the stellar furnaces from which the heavier elements are born, either through fusion or violent explosions.
None of that, however, could happen without HeH+ – a molecule that scientists believe played a huge role in cooling the Universe enough so that the molecular clouds could contract enough to attain the density required to collapse under their own gravity to form the seeds of baby stars.
That’s because HeH+ has a comparatively large separation between its positive and negative charges. In the presence of an electric field, a molecule with a large charge separation undergoes an energy shift that helps dissipate heat, which means HeH+ theoretically played a key role in paving the way for the formation of the first stars.
The researchers performed their experiments at the Max Planck Institute’s Cryogenic Storage Ring, a facility designed to perform experiments in a vacuum environment at temperatures just a few degrees above absolute zero, around -267 degrees Celsius (-449 Fahrenheit), mimicking the conditions of deep space.
There, they carefully studied interactions between HeH+ and a hydrogen atom with one extra neutron in its nucleus, known as deuterium. An interaction between HeH+ and deuterium generates a neutral helium atom and a molecule consisting of one neutral hydrogen atom and one charged deuterium atom (HD+), with lower energy levels than the original components.
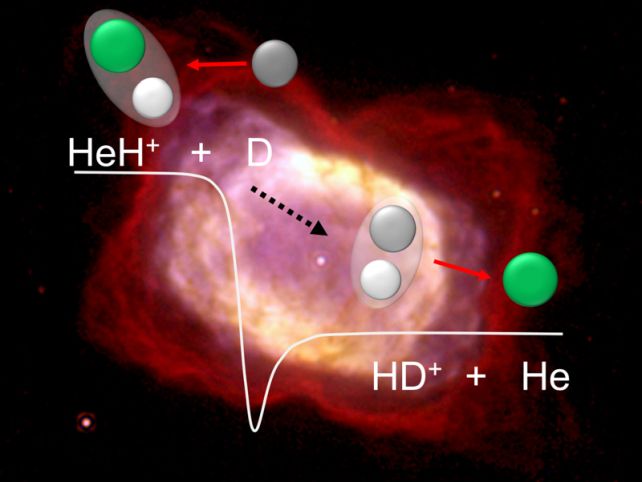
Within the storage ring, the researchers fired two beams of particles; one with HeH+ molecules, the other with neutral deuterium. They changed the speed of the two beams to alter the energy at which the particles collided as a proxy for temperature to see if temperature played a role in the reaction rate.
It did not. The rate at which the reaction took place remained steady, regardless of the proxy temperature – suggesting that the role HeH+ played in the early Universe did not decline as cooling unfolded, and that its role in the formation of the first generation of stars was a significant one.
“Previous theories predicted a major lower within the response chance at low temperatures, however we had been unable to confirm this in both the experiment or new theoretical calculations by our colleagues,” physicist Holger Kreckel from the MPIK explains.
“The reactions of HeH+ with impartial hydrogen and deuterium due to this fact seem to have been much more necessary for chemistry within the early Universe than beforehand assumed.”
The analysis has been printed in Astronomy & Astrophysics.
This web page was created programmatically, to learn the article in its unique location you possibly can go to the hyperlink bellow:
https://www.sciencealert.com/experiment-recreates-the-universes-very-first-chemical-reactions
and if you wish to take away this text from our web site please contact us



