This web page was created programmatically, to learn the article in its unique location you may go to the hyperlink bellow:
https://www.chemistryworld.com/news/first-valence-electron-measurements-capture-fleeting-reaction-process/4022085.article
and if you wish to take away this text from our website please contact us
Chemists at a large x-ray laser within the US have studied a reaction in the most extreme detail yet, watching individual valence electrons as ammonia dissociates. ‘For the first time here, we were able to track just a single valence electron,’ says Ian Gabalski, a PhD candidate at Stanford University in California. This is probably going the primary of many such experiments that may assist scientists to raised perceive the processes of creating the chemical substances which are vital to us.
Valence electrons are an atom’s outermost electrons and outline its chemical behaviour. They ‘are shared between atoms, and so they basically drive all chemical reactions’, emphasises Gabalski. ‘They move on ultra-fast time scales, and they’re additionally overlaying very small distances.’ To measure them requires gentle pulses shorter than the time it takes for the electrons to maneuver. The distance between peaks within the lightwave, higher referred to as the wavelength, should even be much like the scale of the electron orbital. ‘That ends up being an x-ray,’ Gabalski says.
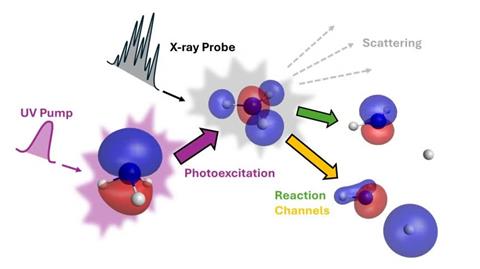
Gabalski was a part of a workforce on the SLAC National Accelerator Laboratory in California led by Mike Glownia and Philip Bucksbaum. Together the researchers used ultraviolet laser gentle to set off particular person hydrogen atoms to separate from ammonia molecules. They then fired x-ray beams from SLAC’s Linac Coherent Light Source (LCLS) on the molecules after an infinitesimally small delay.
Gradually altering the size of the delays, the workforce studied the valence bonding electrons over a couple of hundred femtoseconds. For an concept of how brief that’s, there are 1015 femtoseconds in a second, whereas the universe is about 7 x 1015 minutes previous.
Turning valence electrons on and off once more
Researchers beforehand noticed fleeting glimpses of adjustments in valence electrons in comparable experiments. However, they studied extra advanced molecules, which threw up sudden issues. ‘That short pulse of x-rays ends up taking a picture of all the electrons in your system,’ Gabalski explains. ‘Most of the electrons in the molecule are localised around the heavier atoms, and so they end up being the dominant contribution to the scattering.’
To discover a system the place this drawback didn’t occur, the LCLS scientists labored with Nanna List on the KTH Royal Institute of Technology in Stockholm, Sweden, and the University of Birmingham, UK. List explains that such experiments have been motivated by researchers displaying that it ought to theoretically be doable to make x-ray scattering measurements attributable to valence electrons.
List may use comparable theoretical calculations to ‘decide on a system to target’, specifically ammonia. These calculations had been a part of an in depth proposal the scientists used to use for much-coveted time on the LCLS x-ray laser.
The scientists recorded the adjustments in angle of x-ray beams scattered by ammonia molecules. From that they might work out the place the electrons holding the atoms within the molecule collectively because it adjustments from a pyramidal to a planar form had been, earlier than a hydrogen breaks off.
List’s theoretical fashions helped the LCLS workforce to work out what the valence electron was contributing, turning the impact of its presence on and off. Gabalski explains that their measurements accord with a course of the place the ultraviolet laser causes the valence electron to turn out to be far more delocalised. It is less complicated for hydrogen to dissociate from this state, which lasts for round 100 fs.
Adam Kirrander from the University of Oxford calls the selection of the straightforward goal system intelligent, as a result of it makes it ‘easier to separate the electronic rearrangements from the structural changes in the signal ’. ‘It is a beautiful example of the increasingly accurate mapping of time-dependent dynamics in molecules that is possible,’ he says.
This web page was created programmatically, to learn the article in its unique location you may go to the hyperlink bellow:
https://www.chemistryworld.com/news/first-valence-electron-measurements-capture-fleeting-reaction-process/4022085.article
and if you wish to take away this text from our website please contact us