This web page was created programmatically, to learn the article in its authentic location you may go to the hyperlink bellow:
https://www.scientificamerican.com/article/lab-grown-organoids-could-transform-female-reproductive-medicine/
and if you wish to take away this text from our website please contact us
In 2017, Ashley Moffett, a reproductive immunologist, walked to the pharmacy close to her laboratory on the University of Cambridge, UK, to purchase a being pregnant take a look at. But it wasn’t for Moffett. Her postdoc, Margherita Turco, had created what she thought could be the primary cluster of cells able to mimicking the tissue of the placenta — a placental organoid. But she wanted a means to make certain.
“We must do a pregnancy test on them,” Moffett stated.
If Turco was right, the miniature ball of cells she had created would secrete HCG, the hormone that triggers a optimistic being pregnant take a look at. “I took the stick, put it in, and it was positive,” says Turco, now a reproductive biologist on the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland. “It was the best celebration.”
On supporting science journalism
If you are having fun with this text, contemplate supporting our award-winning journalism by subscribing. By buying a subscription you’re serving to to make sure the way forward for impactful tales in regards to the discoveries and concepts shaping our world immediately.
Scientists make organoids corresponding to this by coaxing stem cells to develop in a jelly-like substance and to self-assemble into clumps of tissue. The sometimes hole or stable balls of cells don’t look something like actual organs. But they do tackle key facets of the organ that they’re meant to signify — liver, brain, lung or abdomen, as an example.
The mini-organs have the benefit of being extra practical than a 2D cell tradition — the traditional in vitro workhorses — as a result of they behave extra like tissue. The cells divide, differentiate, talk, reply to their surroundings and, similar to in an actual organ, die. And, as a result of they include human cells, they are often extra consultant than many animal fashions. “Animals are good models in the generalities, but they start to fall down in the particulars,” says Linda Griffith, a organic engineer on the Massachusetts Institute of Technology in Cambridge.
Over the previous decade, organoid analysis has exploded. Researchers have used them to check early mind improvement, test cancer therapies and rather more. And these 3D fashions stand to grow to be much more essential as US companies, together with the National Institutes of Health, the Food and Drug Administration and the Environmental Protection Agency, goal to maneuver away from animal testing.
Now researchers are utilizing organoids to check feminine replica, an space by which animal fashions could be particularly restricted. Lab mice, for instance, don’t menstruate. And their placentas don’t develop in the identical means as human placentas do. That problem, together with a historic lack of funding for women’s health research, has left fundamental questions unanswered.
“I really see it as a powerful model to do science,” says Mirjana Kessler, a cell biologist on the Ludwig Maximilian University of Munich in Germany, who has developed an organoid that mimics the fallopian tube and a biobank of ovarian most cancers organoids.
Organoids of the placenta, endometrium, ovary and vagina may assist to disclose how these organs operate, and what occurs when issues go awry.
“There’s so much work to do to understand the normal biology,” Turco says.
The placenta invades
The placenta performs a key half in maternal well being throughout being pregnant. Humans aren’t the one species that develops a placenta, however the “human placenta is quite different than most other species, even primates actually, apart from apes”, says Moffett. Mice and people, for instance, each have placentas that invade the uterine lining, however the timing of improvement and the depth of invasion differ. Exactly what occurs through the early days of placental improvement continues to be unclear, however issues at this stage can have severe penalties later.
One of the placenta’s first jobs is to create a hyperlink between the mom and the creating embryo. To do that, the placenta invades the spiral arteries that feed the uterus. The invasive cells open up the arteries, “essentially making a channel so that mom can provide what she needs through her blood supply”, says Victoria Roberts, a developmental biologist on the Oregon National Primate Research Center in Beaverton. (Nature acknowledges that transgender males and non-binary individuals may need feminine reproductive organs and would possibly grow to be pregnant. ‘Mother’ is used on this article to mirror language utilized by the sector.)
The course of could be lethal if it goes fallacious. If the placenta invades too deeply, a situation known as placenta accreta, the expectant mom can lose an excessive amount of blood throughout start. And if the organ doesn’t invade deeply sufficient, then the fetus may not get sufficient vitamins to maintain its progress.
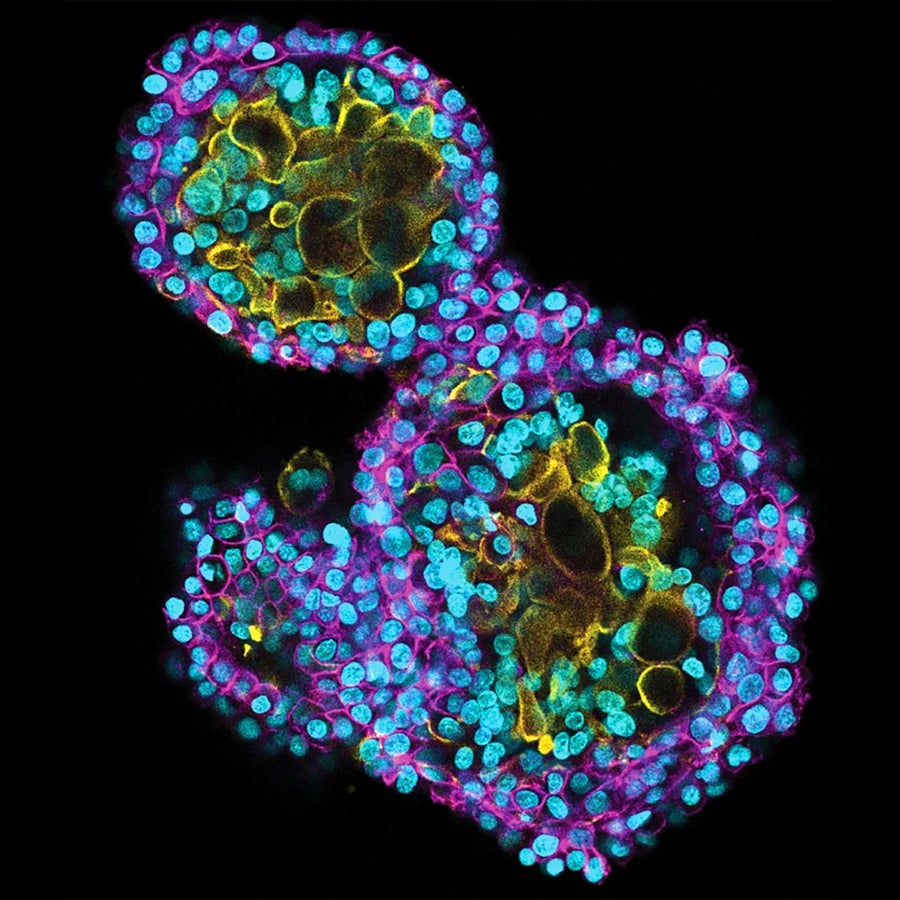
Organoids product of placental cells may also help reveal how the organ invades the uterine lining.
Turco lab, Friedrich Miescher Institute for Biomedical Research
Shallow invasion can even affect the mom’s well being. When the placenta doesn’t get sufficient blood, analysis suggests it could grow to be infected and secrete dangerous components into the mom’s blood that set off pre-eclampsia, a situation characterised by protein build-up within the blood and dangerously hypertension. Worldwide, 2–8% of pregnant individuals develop the situation. “It’s a very serious pregnancy complication that goes silent and undetected until very late into pregnancy,” says Quinton Smith, a chemical engineer on the University of California, Irvine. The solely option to treatment the situation is to ship the child, even when which means a preterm start.
To higher perceive the situation, Smith, Turco and different researchers are utilizing organoids product of placental cells known as trophoblasts to mannequin the molecular processes concerned. Turco is concentrated on the fundamental biology of how invasion is regulated, a course of that appears to be managed by each the fetus and the mom. “It’s got to be a compromise,” Moffett says. “It’s an absolute dialogue.”
That dialogue appears to be occurring between the placenta and the uterine lining. As a working example, when an embryo implants someplace the liner doesn’t exist — on a scar left by a earlier caesarean supply or in a fallopian tube, for instance — “there’s no control of the invasion at all”, Turco says.
Research means that immune cells known as uterine pure killer cells have a key function on this dialog. The cells don’t kill however as a substitute ship out chemical indicators that assist to manage the invasion of the uterine lining.
When Turco, Moffett and their colleagues uncovered the mini-placentas to those chemical indicators and analysed which genes the cells expressed, they discovered that many have been related to pre-eclampsia.
“I’m sure it’s not the whole story,” Moffett says. “But it does show you how you can use those organoids to ask these fundamental questions about human pregnancy.”
Mimicking menstruation
Turco’s first try to create a mini-placenta in 2016 didn’t go as deliberate. The placental tissue she was working with contained not solely trophoblasts, but additionally a number of rogue maternal cells from the endometrium, the uterine lining that builds up after which sheds every month throughout menstruation. Those maternal cells “kept on growing and taking over,” she says. “It was a setback at that time.”
But now Turco sees it as a beautiful discovery, as a result of she as a substitute grew organoids that signify the endometrium. This, together with one other endometrial mannequin printed in the identical yr, actually opened the door for everybody else, says Griffith.
Griffith has been learning the endometrium for greater than a decade. The analysis is private. When Griffith hit puberty, she developed a debilitating situation known as endometriosis. The illness, which impacts about 10% of individuals with a uterus who’re of reproductive age, happens when endometrium-like tissue grows in locations it doesn’t belong.
Because this tissue is trapped contained in the physique, it could’t be shed correctly. Instead, it could irritate surrounding wholesome tissue, inflicting irritation, ache and scar tissue. Although present therapies tackle a few of the signs, they don’t present a treatment.
Organoids are sometimes grown in Matrigel, a jelly-like substance extracted from mouse tumour cells that enables the cells to assemble into 3D constructions. Griffith needed to place epithelial cells, which compose the uterine lining, with stromal cells that assist that lining. In the physique, these cells want to speak with one another to carry in regards to the modifications that happen with the month-to-month cycle. But Matrigel is filled with proteins that may hamper the cell-to-cell communication. So Griffith and her colleagues developed a hydrogel that’s totally artificial.
Griffith’s crew has additionally been engaged on the subsequent step, a mannequin of irregular endometrial tissue that the researchers can use to check therapies for the situation. Because blood vessels are essential to sustaining this tissue, Griffith knew she needed to incorporate them. To do that, she and her colleagues positioned the organoid on a microfluidic chip surrounded by cells that type blood vessels. “We put all of these cells in together at the beginning in a gel, and the blood vessels form spontaneously,” she says. “So the organoids turn into lesion-like structures,” she provides. “It’s actually kind of wild.”
Griffith and her crew have created these mannequin techniques from the cells of a couple of dozen individuals with endometriosis, they usually’re starting to make use of them to check compounds that might be promising therapies for the situation.
Turco, in the meantime, has developed her endometrial organoid right into a mannequin of menstruation. Her crew handled the endometrial organoids with hormones to imitate what occurs when the endometrial lining is regenerating. Then the researchers stopped the hormones to imitate the beginning of menstruation. In the uterus, the liner breaks up naturally. In the mannequin, nonetheless, the researchers break the organoids up mechanically. When the cells are put again right into a gel, the organoids reform. “And you can keep doing this over and over again,” she says.
The mannequin permits them to check the mechanisms at work throughout regeneration. “That’s not possible to study in humans — like ever,” Turco says. Researchers have lengthy thought that the stem cells that lie beneath the floor of the liner are solely liable for regenerating it. But Turco’s analysis means that cells on the floor may need a job, too.
The vagina, ovaries and extra
For Kathryn Patras, a microbiologist at Baylor College of Medicine in Houston, Texas, organoids are a option to discover the diversity of bacteria that colonize the vagina and the way they affect human well being. A wholesome vaginal microbiome may also help to stop dangerous micro organism from taking up. A disrupted microbiome, nonetheless, appears to extend a lady’s threat of catching a sexually transmitted an infection and of experiencing problems throughout being pregnant.
The vaginal microbiome is especially tough to check in mice. Its composition is totally totally different from that of people. And introducing a human microbiome into the mouse vagina is almost inconceivable. Patras tried for years. “It just failed splendidly,” she says.
So Patras and her colleagues harvest naturally present stem cells from the human vagina and coax these cells to type organoids. These mini-vaginas are hole balls, not tubes. And as a result of the researchers try to check the vaginal lining, which isn’t spherical, they break up the organoids to make “open-faced tissue layers”, says Patras. On one facet, the cells have media that nourishes them. On the opposite, “they’re seeing air, which is what they would see in the human tissue,” she says.
One of the crew’s objectives is to have a look at whether or not useful microorganisms which can be discovered sometimes within the vagina, corresponding to Lactobacillus, can defend the vaginal tract from being colonized by dangerous microbes. Although the belief has lengthy been that the pathogens that trigger urinary tract infections come from the intestine, some analysis means that the vaginal microbiome may play a component. Preventing colonization there would possibly cut back the danger of infections within the urinary tract.
Ovaries are additionally getting the organoid remedy, each for learning fertility and the transition to menopause, which comes with a number of aggravating signs and an elevated threat of coronary heart illness, stroke and osteoporosis.
Francesca Duncan, a reproductive biologist at Northwestern University’s Feinberg School of Medicine in Chicago, Illinois, and her colleagues are utilizing ovarian organoids to check reproductive ageing. Researchers on this area have targeted conventionally on the ovary’s follicle. “That’s the kind of functional unit,” says Duncan. It’s the half that generates hormones and incorporates the creating egg. About a decade in the past, nonetheless, researchers in her lab found that, in mice, it’s not simply the egg that ages — the ovary turns into infected and stiffer with age. She suspects that this ovarian ageing may affect each the quantity and high quality of the eggs and, due to this fact, have an effect on fertility.
Duncan needed an in vitro mannequin to check this ageing course of and whether or not medicine would possibly be capable to reverse it. Plenty of labs have managed to develop follicles exterior the ovary. They’ve even managed to get these follicles to offer rise to eggs. But Duncan needed to check the opposite cells that make up the ovary. When a graduate pupil recommended attempting to develop an ovarian organoid, Duncan was sceptical. “It seemed like a fad,” she says. But the scholar was so enthusiastic that Duncan gave the undertaking the inexperienced mild. The analysis has already been “really, really fruitful”, she says.
So far, Duncan’s crew has created ovarian organoids from the ovaries of mice and rhesus macaques, discovering, for instance, that the stiffening of particular person cells within the ovary could be liable for how the ovary tissue stiffens because it ages.
The crew’s subsequent step is to develop human ovarian organoids to display screen compounds that would stave off this stiffening and even reverse it, Duncan says.
Researchers are additionally utilizing organoids to check ovarian most cancers, the fifth-leading reason behind cancer-related deaths in girls. Some groups are learning how the illness emerges by analyzing organoids that mimic the fallopian tube. That’s as a result of analysis means that the overwhelming majority of the deadliest ovarian cancers truly originate there. Other teams are modelling ovarian and different cancers of the feminine reproductive tract by rising organoids from tumour tissue that has been taken from individuals with the illness.
Although researchers are studying a fantastic deal from organoids that signify a single tissue or cell kind, some groups are hoping to study much more by combining them with different organoids or incorporating them into more-complex techniques. Endometrial organoids could be mixed with placental organoids to check a fuller image of invasion, for instance. Or they are often combined with lab-created embryo fashions to check implantation.
Even these more-intricate organoids gained’t seize the total complexity of human tissue. But they don’t should. Organoids could be a reductionist mannequin, however “still they’re revealing so much,” Turco says. “I keep getting surprised.”
This article is reproduced with permission and was first published on September 23, 2025.
This web page was created programmatically, to learn the article in its authentic location you may go to the hyperlink bellow:
https://www.scientificamerican.com/article/lab-grown-organoids-could-transform-female-reproductive-medicine/
and if you wish to take away this text from our website please contact us