This webpage was generated automatically. To view the article in its initial context, you can follow the link below:
https://www.gibsondunn.com/fda-publishes-proposed-rule-on-front-of-package-labeling-what-you-need-to-know/
If you would like to have this article removed from our website, please reach out to us
Client Alert | January 15, 2025
Feedback on the Front-of-Package Proposed Rule must be directed to FDA by May 16, 2025.
On January 14, 2025, the U.S. Food and Drug Administration (FDA) revealed a highly anticipated proposed rule which, if finalized, will mandate front-of-package (FOP) nutritional labeling for food products (FOP Proposed Rule). This proposed regulation is the result of nearly 20 years of deliberation by both the FDA and Congress regarding the necessity and potential formats for a concise FOP nutritional disclosure on food items, aiming to assist consumers in making healthier choices.[2] The formulation of the approach delineated in the proposed rule involved focus groups and testing of diverse formats, including a Guideline Daily Amount (GDA) style similar to the Facts Up Front (FUF) framework created by the industry.[3]
The proposed rule consists of clauses that have been fiercely debated by both the food sector and health proponents, and it is anticipated to encounter considerable administrative and constitutional opposition if ratified by the upcoming Trump administration. Comments on the Front-of-Package Proposed Rule should be sent to Docket No. FDA-2024-N-2910 by the due date of 120 days post-publication, on May 16, 2025.
Here are five key points regarding the FOP Proposed Rule:
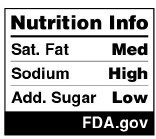
- Introduction of the “Nutrition Info” Panel: The FOP Proposed Rule unveils a new black-and-white “Nutrition Info” panel that will be displayed on the front of food packages. The Nutrition Info panel, illustrated below, provides the serving size and the per-serving percent Daily Value (DV) of three nutrients: saturated fat, sodium, and added sugars. Each of these three nutrients will be categorized as “Low” (5% DV or less), “Med” (6% to 19% DV), or “High” (20% DV or more). Additionally, the panel will include a reference to “FDA.gov,” aimed at enhancing consumer trust and credibility.[4]
 |
FDA additionally permits a smaller variant of the Nutrition Info panel for food items in packages with less than 40 square inches. This compact format displays the Nutrition Facts label in a tabular style, containing solely the “Low,” “Med,” or “High” classifications for the three nutrients to limit, excluding the serving size (or any indication that the classification is based on serving size) or percent DVs for each:[5]
 |
In line with FDA’s existing guidelines for Nutrition Facts, the FDA proposes to exclude food items in packages with a total surface area of less than 12 square inches from featuring the Nutrition Info box.[6] The FOP Proposed Rule also encompasses specialized formats for specific food types, including packages with individually packaged foods intended to be consumed separately, such as variety packs; foods with Nutrition Facts labeling addressing two or more demographics, both per serving and per individual unit amounts, and for the food “as packaged” and “as prepared;” items sold from bulk containers; and, game meats.[7]
- “High,” “Med,” “Low”: After assessing various schemes, FDA has opted to suggest a black-and-white Nutrition Info panel (as opposed to a red/yellow/green traffic light system) that incorporates characterizations of nutrient levels rather than merely numerical information. FDA defended this strategy based on scientific studies revealing that consumers find it challenging to comprehend numeric values in current nutritional labeling when deciding on food choices.[8] The agency indicated that its “Low” and “High” designations align with its historical standards for thresholds for “low” and “high” claims regarding sodium and saturated fats. Although no present regulations exist for “medium” nutrient content claims, the organization is defining “Med” for products that sit between the “Low” and “High” categories.[9] Consistent with this approach, FDA is also proposing revisions to its regulations to allow for low sodium and low saturated fat nutrient content claims in accordance with contemporary nutritional science and the updated Daily Reference Value (DRV) for sodium in the finalized 2016 Nutrition Facts label rule.[10]
- Who is Impacted by the FOP Proposed Rule?: FDA proposes to necessitate that all food products currently marketed to individuals aged 4 and older – the demographic FDA regards as the general population for nutritional labeling – adhere to the Nutrition Info box requirements, unless specific exemptions prevail.[11] The proposed regulation contains exclusions for, among others, foods in packages with a total surface area of less than 12 square inches; packages promoted as gifts comprising a variety or assortment of foods; and, unit containers in multiunit retail food packages.[12]
- When Will the FOP Nutrition Labeling Requirement Become Effective?: If the proposed rule is finalized as stated, it would mandate manufacturers to include a Nutrition Info box on packaged food products three years post the final rule’s effective date (for entities with $10 million or more in yearly food sales) and four years after the effective date (for entities with less than $10 million in yearly food sales). Nevertheless, before the regulation can be finalized, FDA is required to assess any comments regarding the proposed rule and release a final rule. This process may last from one to several years, contingent on the volume and nature of the comments received and the priorities of the agency (and broader administration).
- Making America Healthy Again?: Similar to other recent FDA guidelines and regulations, it remains uncertain whether the FOP Proposed Rule will be finalized considering the change in administration. Nutritional transparency in food labeling is also a key priority for the anticipated leadership of FDA and the U.S. Department of Health and Human Services (HHS) under the new Trump administration. It remains to be seen whether FOP nutritional labeling will be featured as part of FDA’s food regulatory priorities moving ahead.
Interested parties should submit comments to the docket. The FOP Proposed Rule is scheduled for publication in the FederalRegister on January 16, 2025. Feedback regarding the FOP Proposed Regulation must be submitted to Docket No. FDA-2024-N-2910 by the cutoff date of 120 days subsequent to the publication, specifically May 16, 2025. Gibson Dunn stands ready to assist interested stakeholders in assessing the consequences of this proposed regulation, should it be finalized, including via regulatory guidance, FDA and legislative involvement, as well as litigation.
[1] FDA, “Food Labeling: Front-of-Package Nutrition Information,” accessible at https://www.federalregister.gov/public-inspection/2025-00778/food-labeling-front-of-package-nutrition-information (last visited Jan. 14, 2025) (FOP Proposed Regulation). The FOP Proposed Regulation is planned for publication in the Federal Register on Thursday, January 16, 2025.
[2] Refer to id., Part III.B-D.
[3] Refer to id., Part III.D.2-3.
[4] Id., Part I.A, V.B, V.B.2, V.B.5.
[5] Id. Part V.E.5.
[6] Id. Part V.F.2.
[7] Id. Part V.E.1-4, 6-7.
[8] Id., Part III.A, D.2-3.
[9] Id., Part IV.B.3.
[10] Id., Part V.G.
[11] Id., Part V.A.
[12] Id., Part V.F.1-4.
[13] Regulations.gov, Docket No. FDA-2024-N-2910.
Gibson Dunn’s attorneys are available to help answer any inquiries you may have concerning the topics discussed in this update. Please reach out to the Gibson Dunn attorney you typically work with, the authors, or any leader or member of the firm’s FDA & Health Care practice group:
Gustav W. Eyler – Washington, D.C. (+1 202.955.8610, [email protected])
Katlin McKelvie – Washington, D.C. (+1 202.955.8526, [email protected])
John D. W. Partridge – Denver (+1 303.298.5931, [email protected])
Jonathan M. Phillips – Washington, D.C. (+1 202.887.3546, [email protected])
Carlo Felizardo – Washington, D.C. (+1 202.955.8278, [email protected])
Wynne Leahy – Washington, D.C. (+1 202.777.9541, [email protected])
© 2025 Gibson, Dunn & Crutcher LLP. All rights reserved. For contact and additional information, please visit us at www.gibsondunn.com.
Attorney Advertising: These documents were compiled for general informational intents only based on the data available at the moment of publication and are not designed as, do not represent, and should not be regarded as, legal advice or a legal opinion on any particular facts or situations. Gibson Dunn (and its affiliates, lawyers, and staff) shall not bear any responsibility in connection with any usage of these materials. The distribution of these materials does not establish an attorney-client bond with the recipient and should not be depended upon as a substitute for advice from qualified legal counsel. Please remember that facts and situations may differ, and previous outcomes do not ensure a comparable result.
This page was created programmatically, to read the article in its original location you can go to the link bellow:
https://www.gibsondunn.com/fda-publishes-proposed-rule-on-front-of-package-labeling-what-you-need-to-know/
and if you want to remove this article from our site please contact us