This web page was created programmatically, to learn the article in its unique location you possibly can go to the hyperlink bellow:
https://www.nature.com/articles/s41467-025-62088-z
and if you wish to take away this text from our website please contact us
In our preliminary, proof of idea system, we used tetrahydrofuran (THF) as a prototypical solvent, 1 M lithium tetrafluoroborate (LiBF4) and small portions of an alcohol because it was deemed important in direction of the era of a secure SEI that allows N2 and Li+ transport15,20,32,33,34,35. The management experiments confirmed that ammonia solely originate from LiNRR, together with working LiNRR and PMR-LiNRR underneath Ar as an alternative of N2, and PMR-LiNRR with out making use of LiNRR present9. For the aqueous answer, 1 M H2SO4 was used for all of the experiments and Pt was completely used because the anode. We screened the results of alcohol identification utilizing the PMR-LiNRR reactor, utilizing the LiNRR analogue as a reference. In the latter case, solely the alcohol and THF solvent may provide H-atoms whereas within the PMR-LiNRR the water acts in its place H-source as well as. With 0.5% vol. alcohol within the natural compartment and making use of −3 mA cm2 present to both the Pd in each aqueous and natural sides (PMR-LiNRR) or solely to the natural facet (LiNRR), we quantified the Li manufacturing fee (Fig. 2a). We famous that utilizing ethanol resulted within the highest NH3 yield, as quantified through NMR, much like most studies within the LiNRR literature24,36. In some circumstances, the LiNRR system didn’t produce and NH3 and wanted the H-atoms equipped through the PMR facet. In others, NH3 could possibly be detected with out PMR because the small amount of alcohol was enough to supply NH3. It has been noticed that growing the chain size of aliphatic alcohols in LiNRR end result within the noticeable drop in FE, largely assumed that the steric impact of an alcohol impacts the diffusion fee of the proton shuttle throughout the electrolyte and SEI24,36.
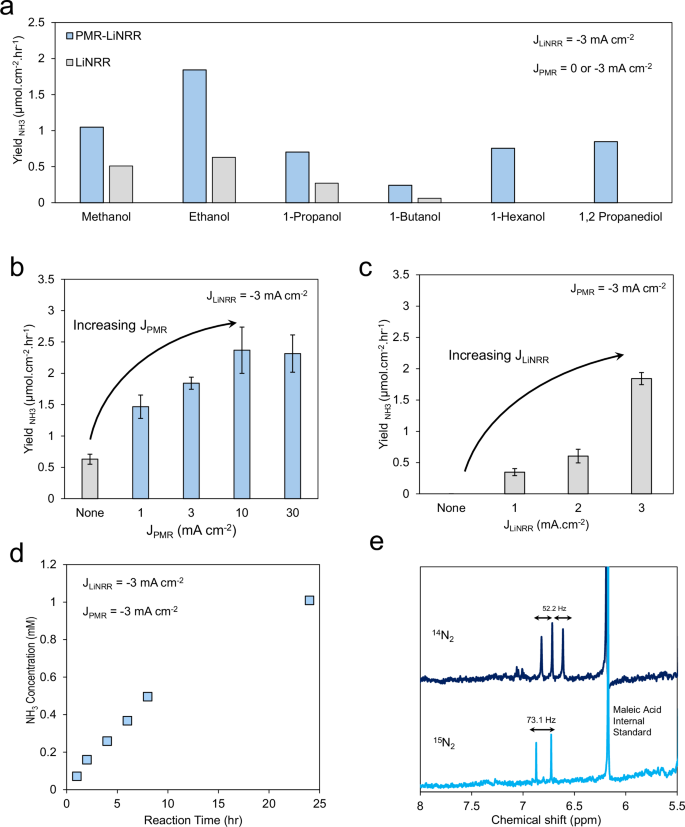
a The results of alcohol identification on the ammonia yield. The software of present to each the aqueous compartment (JPMR) (b) and natural compartment (JLiNRR) (c) had been important to maximise NH3 manufacturing. The system’s efficiency was secure for the primary 24 h, after which the NH3 focus reached a plateau (d). Finally, NH3 manufacturing was verified via the usage of cleaned 15N2, which resulted in a doublet within the HNMR spectrum (e). LiNRR electrolyte was 1 M LiBF4 together with 85 mM alcohol dissolved in THF, and PMR electrolyte was 1 M H2SO4 in H2O. Error bars signify ± commonplace deviation from three unbiased measurements. Source information are offered as a Source Data file.
Keeping with ethanol because the alcohol supply, we famous that growing the utilized present on the PMR facet (whereas maintaining the LiNRR present at −3 mA cm-2) boosted the NH3 manufacturing fee, offering robust proof that H2O was performing instantly because the H-source (Fig. 2b). In addition to this, we additionally famous that, having a steady present on the LiNRR facet was additionally important. When maintaining the PMR present fixed (−3 mA cm−2), growing the LiNRR present additionally resulted in a rise in NH3 manufacturing (Fig. 2c). This illustrates the necessity to repeatedly cut back Li+ to a metallic Li layer which might react with N2 and the usage of the dual-electrochemical system to concurrently drive the PMR and LiNRR course of. We word that we stored the ethanol focus intentionally low. As the focus elevated past 0.5% vol. the ethanol started to more and more function the H-donor and the system behaved extra like the usual LiNRR setup, as evidenced by a diminishing rate-enhancement conferred by the PMR a part of the reactor (Fig. S7). It has been proposed that top ethanol focus disrupts the N2/H+ stability at SEI, wherein ethanol competes with nitrogen to react with metallic lithium, leading to hydrogen evolution somewhat than ammonia manufacturing16,32. Figure S7 reveals that incorporating PMR into LiNRR resulted within the unfavourable shift of optimum ethanol focus. In different phrases, much less ethanol is required to succeed in the N2/H+ stability as a result of further hydrogen injected through PMR.
While our typical response run lasted 1 h, enough to lead to a NH3 focus that may be reliably measured, we subsequent tried to probe the longevity of the preliminary iteration of the PMR-LiNRR setup. When (−3 mA cm−2) was utilized to either side of the Pd foil (PMR-LiNRR), the focus of NH3 repeatedly elevated inside a 24-hr interval (Fig. 2nd) and plateaued afterwards (Fig. S8), doubtlessly as a result of reaching equilibrium within the batch reactor, deactivation of Pd foil or deactivation of SEI. Pd foil throughout PMR sustains bodily deformation presumably as a result of microcracks fashioned by recombination of hydrogen into H237,38. To verify the performance of Pd foil, we periodically examined them for C ≡ N bond cleavage utilizing PMR beforehand reported by our group39 and located that the Pd foils get deactivated roughly 50 to 100 h of PMR-LiNRR operation. In addition to Pd membrane failure, SEI deactivation could play a task right here. SEI is a posh and dynamic layer wherein the construction adjustments over time as proven in Fig. S6.
Finally, to indicate that the NH3 produced resulted from N2 discount versus contamination or different sources, we used 15N2, scrubbed for impurities, because the reagent and produced 15NH3 in comparable portions (Fig. 2e). While our Faradaic effectivity, quantified right here utilizing the entire cost handed via each electrochemical reactors, reached solely modest ranges of as much as 5%, this may be readily improved via the usage of larger pressures of N2, advances in reactor engineering and extra, as has been achieved with commonplace LiNRR methods during the last decade. To perceive the explanation behind the low FE in our work, the basis causes of experimental observations that the N2 stress and ethanol focus have an effect on the LiNRR efficiency must be identified. The LiNRR mechanism consists of various steps, wherein the diffusion of Li+, H+ and N2 species from bulk answer are the bottleneck of the system. Relative to the gradual diffusion steps, the next electrochemical steps are anticipated to be very quick as a result of excessive decreasing potential required for Li electrodeposition. Therefore, N2 and H+ focus instantly have an effect on the LiNRR FE, that are proportional to the N2 stress and ethanol focus, respectively. That’s why to attain excessive FE, excessive N2 stress (>10 bar) is required, which calls for specialised cell design and security tools. On the opposite hand, the current examine focuses on an alternate hydrogen transport pathway. Therefore, to simplify the experimental situations we averted excessive N2 stress.
Our subsequent endeavor entailed acquiring a mechanistic understanding of the PMR-LiNRR course of and offering proof of the perform of the PMR part. To this finish, we took to X-Ray diffraction (XRD) to probe the structural dynamics of the Pd layer. We first acquired an XRD sample of the pre-catalysis XRD foil, which confirmed the anticipated reflections of the Pd construction (Fig. 3a, backside). We subsequent positioned the Pd foil within the PMR-LiNRR reactor and turned on the PMR present solely (60 min, −3 mA cm−2). Taking an XRD sample instantly after this confirmed that the foil consisted of each the Pd and αPdH phases, the latter developed from the electrochemical hydrogenation course of. Next, the foil was positioned again within the PMR-LiNRR reactor and subjected to the LiNRR present solely (60 min, −3 mA cm-2) whereas the PMR present was off. The resultant XRD spectrum confirmed a diminishment of the PdH section and slight shift to bigger 2-theta values because the H atoms partially transferred over to the natural facet and the diploma of hydrogenation throughout the Pd decreased. Continuing the LiNRR facet for an additional 120 min at −3 mA cm-2 resulted within the Pd dropping all of its interstitial H-atoms as they presumably transferred over to the natural facet of the reactor. Note that the XRD spectra had been taken in reflection mode of the portion of Pd going through the aqueous facet. The reverse facet of the Pd confirmed primarily alerts of the air-exposed SEI movie which consisted of a combination of phases together with Pd, PdH0.75, LiF, LiOH, Li2(B12H12), and Li5B4 (Fig. S5). SEI is a posh and dynamic layer wherein the construction adjustments over time as proven in Fig. S6. Our makes an attempt for in-situ remark of SEI confronted technical challenges, primarily as a result of issue of designing a compact electrochemical cell appropriate with our XRD instrument, which required three electrodes and two response chambers with separate circulating electrolytes. If the Pd foil is absolutely lined by a metallic Li layer, the diffusion of H-atoms via lithium turns into an essential issue to be thought-about. However, cryogenic transmission electron microscopy research revealed that metallic lithium formation is just detectable within the absence of ethanol. Without ethanol, a passivating THF-derived SEI kinds on metallic lithium, rendering it inactive for nitrogen discount. Conversely, the presence of ethanol results in a poorly passivating SEI with no underlying metallic lithium33. A current examine utilizing in-situ neutron reflectometry to watch SEI formation revealed that the SEI initially consists of two distinct layers: an internal layer wealthy in lithium-containing inorganic compounds and an outer natural layer. Under excessive present density sustained over lengthy durations like in our system, these layers merge and kind a thicker and extra disordered SEI. This promotes uncontrolled lithium and SEI progress, with inorganic species spreading all through the construction. As lithiation will increase, strains can construct up within the inorganic parts of the SEI, resulting in cracking. These cracks expose contemporary lithium to the electrolyte, triggering additional decomposition reactions and producing further inorganic and natural decomposition species. Such cracking may clarify the merging of the internal and outer layers right into a unified SEI, making them indistinguishable40. Based on these, we assumed that H-atoms leaving the Pd foil don’t essentially diffuse via metallic lithium however could as an alternative be instantly integrated into the SEI.
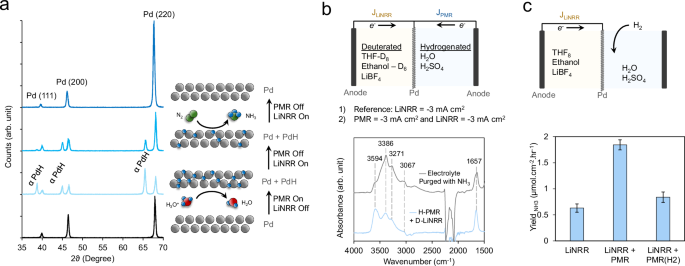
a Dynamics had been first investigated via XRD measurements following the applying of present within the PMR and LiNRR parts. b The H-source for NH3 synthesis was investigated through IR spectroscopy measurements of post-electrolysis options when the LiNRR half was absolutely deuterated. c H2 was proven to be an alternate supply of *H species that might hydrogenate the LiNx layer, however this route was much less efficient than direct electrochemical *H era. Error bars signify ± commonplace deviation from three unbiased measurements. Source information are offered as a Source Data file.
The subsequent demonstration of the PMR-LiNRR system entailed the usage of infrared (IR) spectroscopy to elucidate the hydrogen supply for NH3 manufacturing. To execute this, we modified the PMR-LiNRR setup such that deuterated variations of THF and ethanol had been completely used within the LiNRR facet whereas the aqueous PMR facet was stored to hydrogenated H2O/H2SO4 (Fig. 3b). We ran an experiment however with each the LiNRR and PMR currents on (60 min, −3 mA cm−2) measured the absorbance of the post-electrolysis answer on the LiNRR facet. An improve within the absorbance options of the N-H bands was evident as a result of NH3 within the electrolyte that was produced utilizing H2O because the H-source. The bands matched these of a THF answer that was purged with NH3 fuel. In earlier studies, it has been proven {that a} appreciable quantity of ammonia will get trapped in SEI matrix16. Since the hydrogen diffusion path is totally different in our examine in comparison with the earlier studies, it may be assumed {that a} appreciable quantity of hydrogen will get trapped on the again facet of the SEI going through the Pd foil. Therefore, to qualitatively deal with the difficulty, we analyzed SEI by dissolving it into methanol. The relative abundance of N-D over N-H was calculated to be 0.785 (Fig. S14).
As a complementary proof-of-concept we additionally confirmed that the LiNx layer on the Li-NRR facet will be hydrogenated with out the necessity for electrochemical steps on the PMR facet. To this finish, we disconnected the PMR electrochemical cell and as an alternative bubbled H2 via the H2SO4 electrolyte. In this case, the H2 may spontaneously dissociate into 2H* species and equally diffuse via the Pd membrane to hydrogenate the LiNx layer. Using this methodology, we additionally recorded a measurable improve within the NH3 manufacturing fee, although not as excessive as when a continuing present was utilized on the PMR facet (Fig. 3C). This is probably going as a result of larger fee of *H formation from electrochemical H3O+ discount at 3 mA/cm2 as in comparison with H2 dissociated from H2 dissolved in an aqueous electrolyte. Thus, these experiments illustrate the synergy of the PMR-LiNRR system wherein Faradaic reactions on either side are mandatory however perform complementary reactions of Li+ discount, H3O+ discount and subsequent *H switch. Additionally, we tried an natural proton supply in PMR chamber as an alternative of aqueous H2SO4, which as anticipated confirmed improved efficiency in comparison with LiNRR (Fig. S15).
In this work, we purposely stored to very commonplace parameters with minimal modifications to easily display the idea of enhancing Li-NRR with a PMR reactor. While the efficiency of this technique, by way of throughput or Faradaic effectivity, doesn’t compete with the state-of-the-art, there’s loads of unexplored avenues to additional enhance this. For instance, growing the stress to the 20 Bar generally utilized in Li-NRR methods stands to spice up reactivity as N2 focus is elevated15. Further, exploring nanostructuring/thickness of the Pd or components like H2O, O2 and different solvent/electrolyte molecules to modulate SEI properties are different readily pursuable observe up endeavors19,20,41,42.
Looking forward, whereas Li is used within the area due to the useful SEI that kinds on the electrode floor in LiNRR methods however in principle different metals may also be used43. In follow, Ca44 and Mg45 had been not too long ago demonstrated as viable alternate options to Li. Other metals like Al have additionally been hypothesized to be energetic, although a considerable problem lies within the formation of an SEI that’s sufficiently conductive and porous in direction of N2, H-atom donors and NH343. We imagine that the growth of the M-NRR + PMR idea could allow the usage of such metals as a result of H-transfer turns into decoupled. The PMR-LiNRR has its personal intrinsic limitations, notably low N2 dissolution and incompatibility with GDE. The work is in progress to deal with on this entrance by modifying the response setting and geometry. Another limitation of PMR-LiNRR is the usage of palladium because the lithium plating substrate, which might result in the formation of Pd-Li alloy and degrading the substrate floor46,47. Moreover, hydrogen diffusion via palladium can result in recombination into molecular hydrogen (H₂), inflicting microcrack formation throughout the palladium foil. This course of can deform the fabric and compromise its long-term structural integrity37,38.
In all, this work demonstrates that H-atom switch and metal-mediated NRR will be decoupled via the mixing of a PMR reactor with a standard MNRR setup. Such a singular geometry stands to convey potential advantages to the efficiency of M-NRR methods because the transport constraints of the SEI layer are relaxed. While this work introduced on modest enhancements and general efficiencies nonetheless beneath the state-of-the-art, we anticipate developments within the chemistry and engineering of PMR-MNRR methods could convey this expertise nearer to sensible viability.
This web page was created programmatically, to learn the article in its unique location you possibly can go to the hyperlink bellow:
https://www.nature.com/articles/s41467-025-62088-z
and if you wish to take away this text from our website please contact us



