This web page was created programmatically, to learn the article in its unique location you’ll be able to go to the hyperlink bellow:
https://www.scientificamerican.com/article/human-embryo-implantation-revealed-in-first-ever-3d-images/
and if you wish to take away this text from our website please contact us
August 15, 2025
3 min learn
First 3D Images of Human Embryo Implantation Reveal New Details of the Process
Analyzing embryo actions in uteruslike environments may supply clues to bettering the success fee of in vitro fertilization
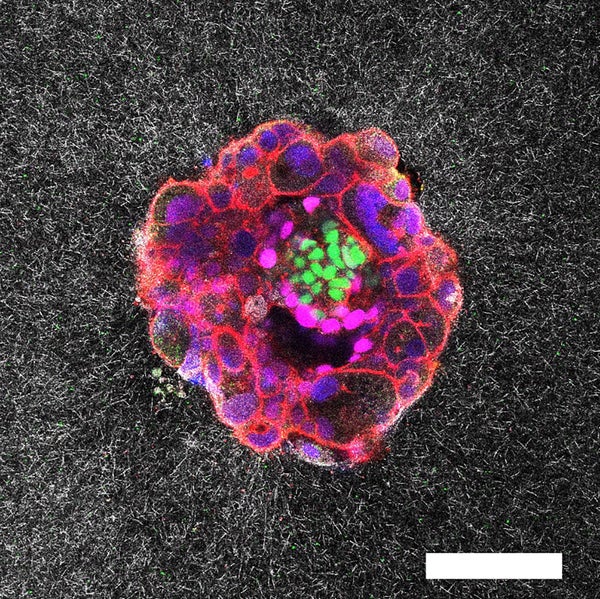
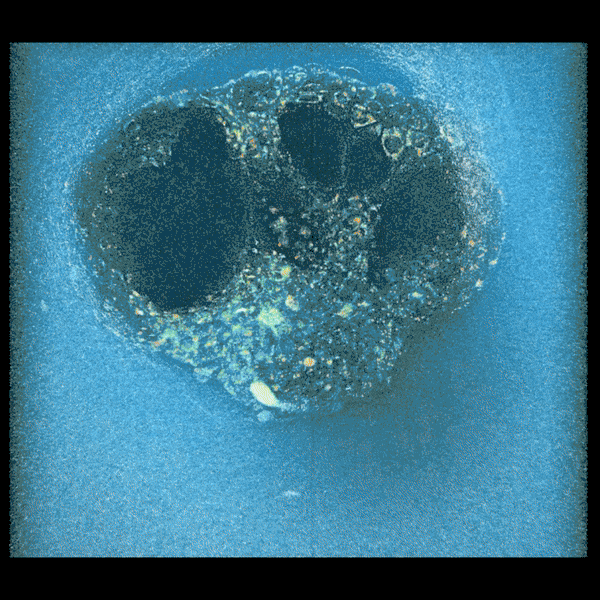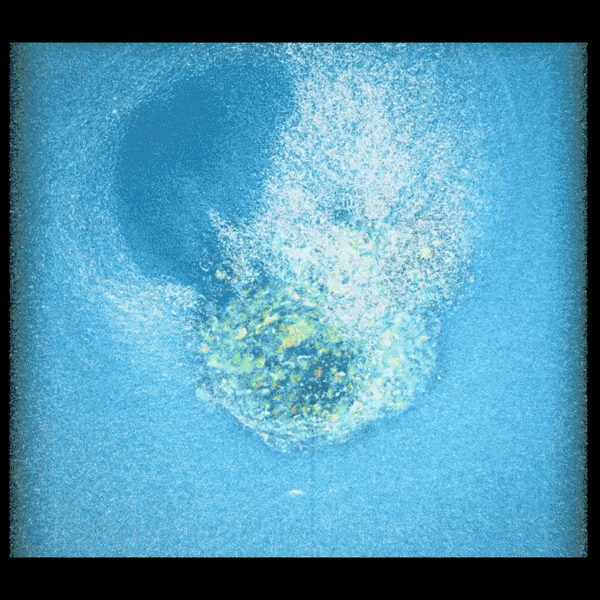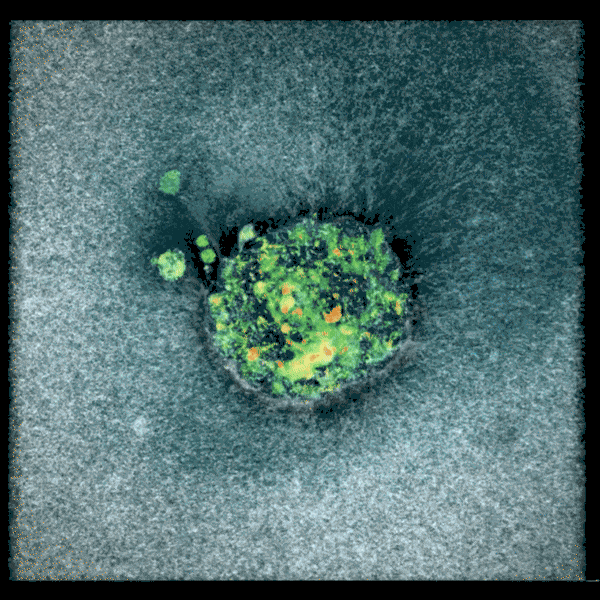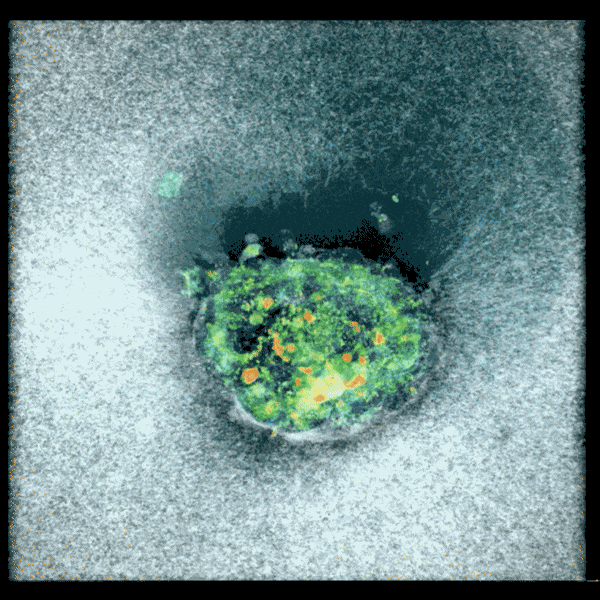
Confocal microscopy picture of a nine-day-old human embryo. Specific proteins and mobile buildings have been colored within the picture: OCT4 (inexperienced), which is expounded to embryonic stem cells; GATA6 (magenta), which is related to early tissue formation; DAPI (blue), which marks the DNA within the nuclei; and phalloidin (pink), which reveals the actin cytoskeleton. The scale bar corresponds to 100 µm.
Institute for Bioengineering of Catalonia (IBEC)
Researchers have captured the very first real-time, three-dimensional images and videos of a human embryo implanting into collagen designed to imitate uterine tissue —a key stage in copy. The ensuing footage, which exhibits how embryos push and pull to anchor themselves within the uterus in vivid element, may result in enhancements for in vitro fertilization (IVF) strategies, the scientists say.
“This will allow us to develop treatments specifically targeting implantation, which is the biggest roadblock in human reproduction,” says Samuel Ojosnegros, a bioengineer on the Barcelona Institute of Science and Technology in Spain and a co-author of the brand new examine, which was printed in Science Advances.
Five days after an embryo is fertilized artificially, fertility docs should implant it into the physique so it could actually proceed to develop. “What happens between the transfer and the first ultrasound weeks later is a black box,” says Ojosnegros, who can be co-founder of the biotech firm Serabiotics. Implantation failure is likely one of the fundamental causes of infertility —as much as 60 % of miscarriages happen throughout this course of.
On supporting science journalism
If you are having fun with this text, take into account supporting our award-winning journalism by subscribing. By buying a subscription you’re serving to to make sure the way forward for impactful tales in regards to the discoveries and concepts shaping our world at this time.
The first successful culture of human embryos beyond implantation was demonstrated in a petri dish in a lab in 2016, however Ojosnegros and his workforce wished to see what this course of would seem like in 3D tissue that was extra just like that of the uterus.
To do that, the workforce designed a particular ex vivo system fabricated from gel and collagen—a protein discovered within the uterine lining—and used embryos donated by individuals who had accomplished an assisted copy course of. The system works, Ojosnegros says, as a result of the community of collagen fibers alerts to the embryo at a molecular degree that this can be a pure matrix.
By utilizing superior 3D microscopes, the researchers recorded the motion over time. Tracking tiny actions within the gel’s fibers allowed them to map precisely the place and the way strongly the embryos had been pulling. The researchers did the identical with mouse embryos to match motion patterns.
The footage confirmed that human embryos generate a community of tiny pulling forces that ripple by means of the womb. They burrow into the encircling tissue from one aspect, creating a number of small traction factors that tug the liner in all instructions. Mouse embryos, then again, unfold out extra throughout the floor and pull primarily alongside two or three sturdy strains.
Embryo compacting and invading the uterine tissue.
When the researchers utilized exterior rigidity to the matrix, tugging it with tiny forceps, they observed the embryos reoriented towards these areas. The scientists recommend micro contractions could be guiding the embryo to implant within the optimum path within the uterus. “We believe these micro contractions are what the embryo uses to guide itself toward the blood vessels and the nutrients it needs,” Ojosnegros explains, including that extra research are wanted to verify this speculation.
In each mouse and human experiments, the power and sample of those forces had been linked to the embryo’s well being, which means embryos that pulled much less had been much less more likely to efficiently invade the tissue. Observing implantation in real-time in a 3D mannequin is a “quantum leap” in contrast with the two-dimensional observations that exist already, says developmental biologist Claudia Spits of the Free University of Brussels, who was not concerned within the analysis. Keeping an embryo alive beneath these situations is extraordinarily troublesome, she says. “What you see in a 10-second video is years of setting these [conditions] up so that the embryo can survive,” Spits provides.
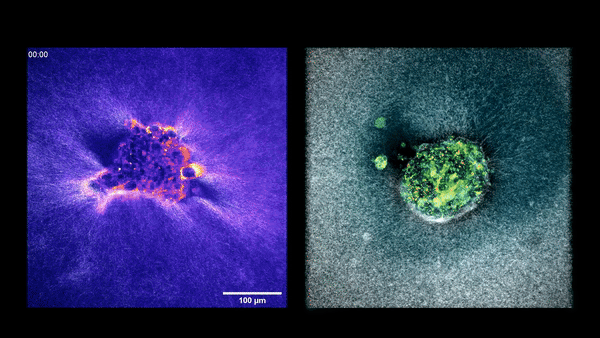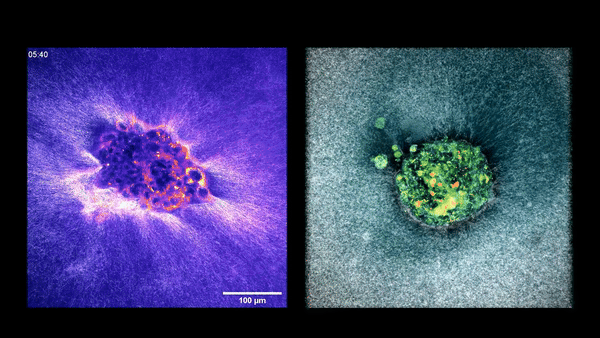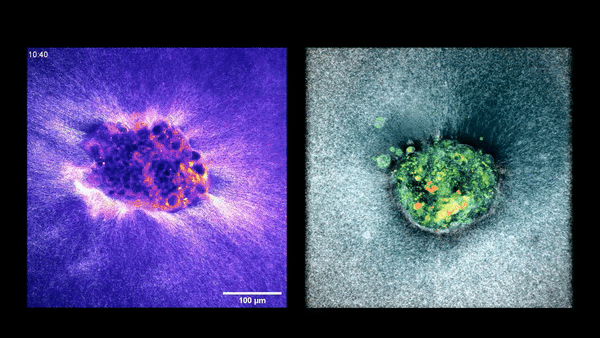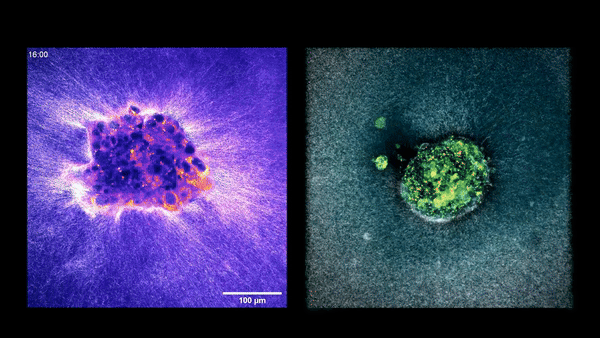
Two embryos implanting into the uterus.
“This study sets the stage to explore the dynamics of implantation in unprecedented detail,” says Magdalena Żernicka-Goetz, a developmental biologist on the California Institute of Technology, who was not concerned within the analysis. The findings add to the rising physique of labor on human postimplantation observations printed within the final 9 years, she says, and “these studies are a thrilling step forward in understanding a stage of human development that has long been hidden from view.” Future analysis, Żernicka-Goetz notes, continues to be wanted to match how embryos behave throughout completely different “uterus-like” platforms to see whether or not developmental trajectories differ.
The matrix developed by Ojosnegros’s workforce just isn’t meant for in vitro fertilization procedures, however it might be a useful device for pharmaceutical corporations and laboratories testing serums or several types of embryos. “By beginning to understand how the embryo behaves,” Ojosnegros says, “we can start thinking about the future possibility of selecting healthy embryos or those more capable of implanting.” Spits stays skeptical of that assertion as a result of replicating this expertise in different laboratories might be a serious problem. But she says the outcomes are a “major step forward” in tech that would have future functions as soon as different laboratories are in a position to do their very own 3D implantations.
This web page was created programmatically, to learn the article in its unique location you’ll be able to go to the hyperlink bellow:
https://www.scientificamerican.com/article/human-embryo-implantation-revealed-in-first-ever-3d-images/
and if you wish to take away this text from our website please contact us



