This web page was created programmatically, to learn the article in its authentic location you’ll be able to go to the hyperlink bellow:
https://www.chemistryworld.com/news/magnets-could-be-simple-way-to-enhance-oxygen-generation-on-a-mission-to-mars/4022041.article
and if you wish to take away this text from our website please contact us
Commercially out there magnets may considerably enhance the effectivity of water splitting in a microgravity surroundings. These kinds of gadget could be used to produce astronauts with oxygen throughout long-term missions however they’re much less efficient in low gravity. The worldwide workforce of researchers, from the US, UK and Germany, who made the invention, additionally developed two proof-of-concept gadgets which they declare may, with additional testing in low gravity environments, be utilized in future house missions.

Ensuring astronauts have entry to a dependable and steady provide of oxygen throughout deep house missions has been a problem because the early days of house exploration within the Sixties. Currently, in microgravity environments oxygen is produced utilizing electrochemical water-splitting, which requires advanced mechanical parts and a major quantity of vitality. The course of is additional sophisticated by the truth that within the weightlessness of microgravity, fuel bubbles don’t float upwards like they do on Earth. Instead, they have an inclination to stay to the floor of the electrode, inhibiting the response.
Putting Mars inside attain
To handle these challenges, the workforce got down to engineer electrochemical cells that might assist to simplify the best way oxygen is generated in house. ‘In the case of a Mars transit mission, the reliability of the oxygen production system … is still not high enough to support a long-term mission,’ explains Álvaro Romero-Calvo, an aerospace engineer on the Georgia Institute of Technology and a member of the workforce. ‘The problem is that there are so many moving components and centrifuges and pumps and hydrogen sensors and so on in a closed loop operating in reduced gravity, that all those spare components stack up and add a lot of mass to your system.’
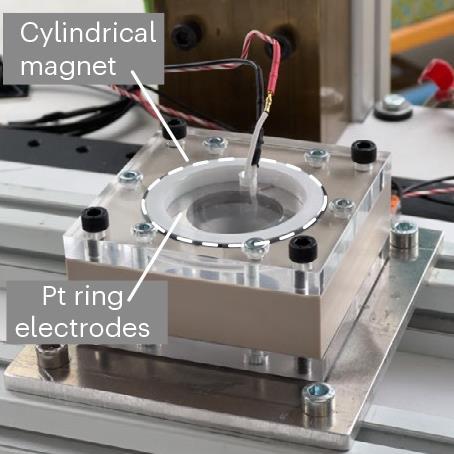
Incorporating off-the-shelf neodymium magnets into electrolysis gadgets, the workforce developed a passive section separation system that pushed the bubbles away from the electrodes and picked up them at designated spots. Romero-Calvo explains that there are two foremost forces being demonstrated of their work: diamagnetic pressure and magnetohydrodynamics. The magnetohydrodynamic impact works to enhance fuel bubble detachment from the electrode floor inflicting them to swirl round, whereas the diamagnetic impact helps to direct the fuel bubbles to particular assortment factors.
‘What we’re finally attempting to do is to separate fuel bubbles – oxygen and hydrogen – from the water or the electrolyte, with out transferring components or centrifuges or pumps or something like that,’ says Romero-Calvo.
To examine the influence of those two forces the researchers used a drop tower to generate temporary intervals of microgravity throughout free fall lasting a complete of 9.3 seconds. Using this method, they discovered that the magnet enhanced water electrolysis with present density enhancements of as much as 240% in microgravity, in contrast with electrolysis gadgets and not using a magnet.

To exploit these results, the researchers developed two easy proof-of-concept gadgets – one a proton-exchange membrane electrolyser cell that makes use of diamagnetic forces for environment friendly oxygen and hydrogen fuel assortment and the opposite a magnetohydrodynamic drive cell that causes vortical fuel–liquid section separation. In microgravity, they discovered that the gadgets achieved water splitting with an effectivity near that achievable on Earth.
‘The goal of these two proof-of-concept devices is, can we induce gas separation in microgravity to produce oxygen and separate it within the same device … in a very simple way?’ says Romero-Calvo.
Long-term testing
The workforce at the moment are seeking to assess the long-term efficiency of the system. ‘We need long-term microgravity conditions, either through a suborbital rocket, which is something we’re going to launch within the close to future, or via orbital experiments,’ he provides. ‘The second part is that many innovations that happen at the electrode or the cell level … work very well in a tiny, little cell but when you try to make it fit for astronauts for six months, it doesn’t actually work that properly. So, the size up is one thing we’re engaged on as properly.’
Mark Symes, an electrochemist on the University of Glasgow, described the work as ‘super cool’ and ‘a tour de force’ of doing electrolysis in troublesome situations. ‘Electrolysis in space is a huge issue, particularly if you’re going to go and dwell or have a semi-permanent base on the moon or Mars, you’re not going to need to ferry oxygen backwards and forwards from Earth endlessly,’ he explains.
‘What’s actually cool about this magnetic work, is there are not any transferring components, so that you simply have an ordinary electrolyser with a magnet. Basically, the magnetic area is producing a convection and a form of jitter that pushes these bubbles off earlier than they get too massive.’
However, Symes says he was left with a number of questions concerning the work. ‘I didn’t see, a minimum of in the principle paper, that they measure the purity of these gases, and that’s clearly one thing that you’d need to do; you’d need to guarantee that your stage of hydrogen and your oxygen was under the explosion restrict, in any other case you wouldn’t need to put that on the spacecraft.’
‘The other question I had was around their current density, so the rate at which they can make gas per unit area of electrode,’ he provides. ‘For both the devices … they’re very a lot in the direction of the decrease finish of what you’d need – 150 milliamps per centimetre squared. It’s not nice – for reference, a traditional electrolyser on Earth would run a minimum of an amp per centimetre squared, so a minimum of 10 to twenty occasions extra. So, you’d need to enhance that present density while conserving the gases separate.’
This web page was created programmatically, to learn the article in its authentic location you’ll be able to go to the hyperlink bellow:
https://www.chemistryworld.com/news/magnets-could-be-simple-way-to-enhance-oxygen-generation-on-a-mission-to-mars/4022041.article
and if you wish to take away this text from our website please contact us


